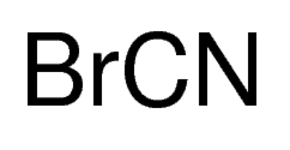A Division Of


Cyanogen bromide
CAS No. 506-68-3 | Catalog No. 655608
Synonyms
Bromine cyanide; Bromine monocyanide; Bromocyanide; Bromocyanogen; Campilit; Cyanic bromide; Cyanobromide; Cyanogen bromide; Cyanogen monobromide
Properties
| Boiling Point | 61 – 62 ºC |
| Form | Crystalline white solid; Custom Solutions (Acetonitrile, Toluene, etc…) |
| Melting Point | 50 – 53 ºC |
| Molecular Weight | 105.92 g/mol |
| Specific Gravity | 2.015 |
News & More

January 22, 2025
FAR Chemical to Exhibit at the SOCMA Tradeshow in Nashville

February 28, 2023