A Division Of


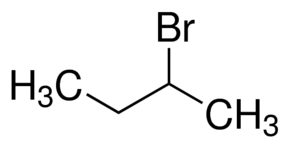
2-Bromobutane
CAS No. 78-76-2 | Catalog No. 200025
Synonyms
2-Bromobutane; (±)-2-Bromobutane; (±)-sec-Butyl bromide; 2-Butyl bromide; Methylethylbromomethane; sec-Butyl bromide
Properties
| Boiling Point | 91 °C |
| Form | Liquid |
| Melting Point | -112 °C |
| Molecular Weight | 137.02 g/mol |
| Specific Gravity | 1.243 |
News & More

January 22, 2025
FAR Chemical to Exhibit at the SOCMA Tradeshow in Nashville

February 28, 2023